Key RELiZORB® clinical accomplishments
RELiZORB initially received de novo approval based on pre-clinical data. De novo approval was followed by published short and long-term clinical efficacy and safety data that expanded the RELiZORB label to include patients down to 5-years of age.1,2 CMS listed a unique B-Code and KOLs published a consensus statement supporting greater patient access to RELiZORB as standard of care for patients with cystic fibrosis on enteral feeding. In August 2023, the RELiZORB label was expanded again to include pediatric patients as young as 2 years old.
RELiZORB is indicated for use in pediatric patients (ages 2 years and above) and adult patients to hydrolyze fats in enteral formula. RELiZORB is for use with enteral feeding only; do not connect to intravenous or other medical tubing. Medications should not be administered through RELiZORB. For more information about RELiZORB, including Instructions for Use and Patient Guide, please visit RELiZORB.com.
Pre-Clinical Animal Studies
The results from animal studies demonstrate that when iLipase® or RELiZORB are used to hydrolyze fats in enteral formulas prior to ingestion, the pre-hydrolyzed formulas are well-tolerated. Observation of study subjects suggests that there is no safety risk resulting from the use of iLipase or RELiZORB to hydrolyze fats in enteral formulas. In addition, these animal studies indicate that use of iLipase or RELiZORB to hydrolyze fats in enteral formula results in increased plasma concentrations of long-chain fatty acids.
RELiZORB pre-clinical animal studies
This test was performed to evaluate the absorption of LCPUFA from infant formula when pre-hydrolyzed with microbial lipase, used in RELiZORB. The effect of soluble lipase, divided between 4 daily feedings, was tested in young growing EPI pigs. There were 4 animals in the lipase group and 4 controls. The animals were fed NAN® (Nestlé®) formula, followed by NAN formula enriched with long-chain triglycerides containing LCPUFAs DHA (1%) and AA (2%). After randomization they were switched to feeding with enriched formula pre-hydrolyzed with 1,300 units/day of lipase. The effect of pre-digestion was monitored by reduction of total and LCPUFA fecal fats, change in coefficient of fat absorption (%CFA), together with increase in AA and DHA levels in plasma and tissues such as liver, retina, heart, and fat. The results of this study demonstrated no mortality, adverse clinical signs, or pathologic macroscopic findings along the gut or in the liver following the seven days of administration of pre-hydrolyzed formula. Both groups demonstrated a reduction in fecal fats and an increase in fat absorption in all tested tissues. Complete study results on file at Alcresta.
A 6-week study was conducted in seventeen (17) EPI pigs and six (6) control animals fed 4 times daily with formula containing pre-hydrolyzed fat after exposure to the iLipase in RELiZORB. The results from this long-term study demonstrated that EPI pigs fed pre-hydrolyzed fat after exposure to the iLipase had total fat content in stool reduced by 43%, thereby reducing steatorrhea when compared to non-hydrolyzed formula. The EPI pigs fed pre-hydrolyzed formula had improved digestion of LCPUFAs resulting in accretion of DHA in the heart, liver and adipose tissue, as well as improved absorption of fat soluble vitamins. Fasting levels of triglyceride, cholesterol, HDL, and LDL in EPI pigs fed formula with pre-hydrolyzed fat were similar to values in the healthy controls. Histopathology analysis of tissues collected at the end of study demonstrated no evidence of toxicity with the consumption of pre-hydrolyzed fat from formula with 4 g of iLipase/day when compared to non-hydrolyzed formula. Overall, this study demonstrated that the consumption of pre-hydrolyzed fat after exposure to the iLipase in a novel point-of care product enhanced fat absorption, normalized blood lipid profile, and increased blood and tissue levels of DHA and AA, while reducing findings of gastrointestinal dysfunction typically seen in people with CF (e.g. steatorrhea). Complete study results on file at Alcresta.
This was a 12-day study that enrolled eleven (11) EPI pigs. Five (5) EPI pigs in the control arm were fed with non-hydrolyzed Peptamen AF® via a G-tube and six (6) EPI pigs in the RELiZORB arm were fed Peptamen AF pre-hydrolyzed by RELiZORB via a G-tube. Pigs were fed during the day with a standard solid feed similar to the average human high fat diet (~1400 kcal/day/pig). In order to mimic nighttime enteral feeding and the intended use in EPI patients, the EPI pigs were supplemented with an additional 750 calories (500 mL; 1.8 g Omega 3, Peptamen AF, Nestlé® Nutrition, EU), nightly delivered with a flow rate of 2 mL/minute over 4 hours via G-tube feeding. Peptamen AF is a semi-elemental enteral formula that provides pre-hydrolyzed protein, and so the use of PERT for protein digestion was unnecessary and therefore not provided. Results from this study demonstrated that RELiZORB, when used per the intended use, was safe and well-tolerated and reduced signs of gastrointestinal dysfunction typically seen in individuals with EPI (eg. steatorrhea). The 12 days of nighttime feeding with RELiZORB resulted in decreased fecal fat losses and increased plasma levels of DHA and EPA with corresponding tissue accretion of DHA and EPA as well. Complete study results on file at Alcresta.
A study in EPI pigs was performed to measure the effect of RELiZORB on fat absorption. Fat absorption was evaluated by measuring important plasma LCPUFAs, specifically omega-3 fatty acids DHA and EPA, over 24 hours. The study consisted of three groups: an EPI group (n=6) receiving formula hydrolyzed with RELiZORB, a second EPI group (n=5) receiving non-hydrolyzed formula without RELiZORB, and a third healthy control group (n=3) with normal digestive function receiving non-hydrolyzed formula without RELiZORB. Peptamen AF®, a semi-elemental enteral formula containing hydrolyzed protein, was provided over 4 hours (500 mL; pump rate 120 mL/hour). Fat absorption was evaluated by measuring plasma DHA and EPA levels. RELiZORB use was well tolerated in the porcine model with normal food intake and no serious adverse reactions. No vomiting or diarrhea was recorded during the 24-hour period. RELiZORB use in subjects with EPI resulted in normalized plasma levels of DHA and EPA compared with the levels in the healthy control group. Formula hydrolyzed with RELiZORB was associated with a statistically significant increase in total fat absorption and improvement in uptake of omega-3 fatty acids (DHA and EPA) in plasma levels over 24-hours compared with non-hydrolyzed formula without RELiZORB (p < 0.05). Complete study results on file at Alcresta.
ALC-078 pre-clinical animal studies
This test was performed to evaluate the development of ALC-078 to improve fat and nutrient absorption in a porcine model of short bowel syndrome (SBS) on continuous enteral feeding. SBS piglets gain less weight, develop fat malabsorption, and demonstrate a decrease in fat-soluble vitamin concentrations, representing important clinical conditions in the pediatric and adult SBS populations not shown in other preclinical studies. The three-arm study assessed fifteen male piglets. The animals were randomized to no intestinal resection (n=5), 75% resection (n=5), or 75% resection plus ALC-078 (n=5). After recovery, the animals were treated for 14 days. Piglets received 60% of nutrition from continuous enteral nutrition (EN) and 40% from chow. The degree of fat malabsorption was determined based on the coefficient of fat absorption (CFA). Body weight, fat-soluble vitamins, and nutritional markers were assessed. The study showed that adverse events were similar across the three groups and therefore not related to ALC-078, and all groups received similar durations of antibiotic treatments. ALC-078-treated animals had similar weight gain compared to resected piglets. The mean CFA was higher in the ALC-078 group compared to the untreated resected group but the difference did not reach statistical significance (87.1% vs. 79.3%, P = 0.19). ALC-078-treated animals had increased concentrations of vitamin D and vitamin E, improved HDL concentration, and a decrease in serum triglyceride concentrations. In conclusion, ALC-078 showed an increase in the absorption of fat-soluble vitamins and may improve fat malabsorption. Complete study results on file at Alcresta. For access to the full text of the study publication, Tsikis, ST, et al., An in-line digestive cartridge increases enteral fat and vitamin absorption in a porcine model of short bowel syndrome, visit Clin Nutr 2022:41: 1093-1101.
The purpose of this study was to evaluate the safety and efficacy of the ALC-078 development device when used in conjunction with bolus enteral feeding in weaning parenteral nutrition in a porcine model of short bowel syndrome (SBS). Eleven six-week old male piglets were utilized in this study. Animals were randomized to 75% intestinal resection (control, n=6) or 75% intestinal resection + ALC-078 (treatment, n=5). Parenteral nutrition (PN) was initiated post-operatively and was decreased as enteral nutrition (EN) was advanced. EN was delivered daily via 6 bolus feedings with or without ALC-078. Animals were studied and maintained for 14 days. The change in EN/PN calories and biochemical markers were compared across groups by time-weighted area under the curve. Intestinal adaptation was assessed by the growth in bowel length, plasma GLP-2 concentration, and Ki67 immunostaining to determine cell crypt proliferation. The study showed that ALC-078 piglets received similar calories and had similar weight gain compared to controls (P=0.43). ALC-078 animals had a 19% greater reduction in PN Calories (95% CI: 12-27%, P=0.0002) and demonstrated a high average EN advancement (66 vs. 47% of total calories, P=0.0008) during the 14-day experiment. Intestinal crypt cell proliferation was 1.9-fold higher in ALC-078 piglets compared to controls (41.9 vs. 22.4 Ki67+ cells/crypt, P=0.02) which was accompanied by a significant increase in intestinal length (19.5% vs. 0.7%, P=0.03). Animals treated with ALC-078 also trended towards higher plasma GLP-2 concentrations at day 15. Despite significantly lower PN requirements, ALC-078 animals had similar concentrations of albumin and other nutritional markers compared to controls. In conclusion, ALC-078 showed a reduction in PN dependence, increased EN advancement, and an increase in intestinal length, an indicator of intestinal adaptation. Complete study results on file at Alcresta. For access to the full text of the study publication, Tsikis, ST, et al., A Digestive Cartridge Reduces Parenteral Nutrition Dependence and Increases Bowel Growth in a Piglet Short Bowel Syndrome, visit Ann. Surg. 2023.
Human Clinical Studies
RELiZORB clinical trials now enrolling patients
- 90 day, Phase 3, open labeled exploratory study of RELiZORB: Learn more by visiting ClinicalTrials.gov, trial NCT03530852
- 90 day observational study as an extension to the Phase 3, open labeled exploratory study of RELiZORB: Learn more by visiting ClinicalTrials.gov, trial NCT05635747
RELiZORB is the only clinically-studied and FDA-cleared product addressing fat malabsorption in enteral nutrition. Extensive clinical data supports the benefits and versatility of RELiZORB in cystic fibrosis.
24-Hour Study1
(P<0.001)
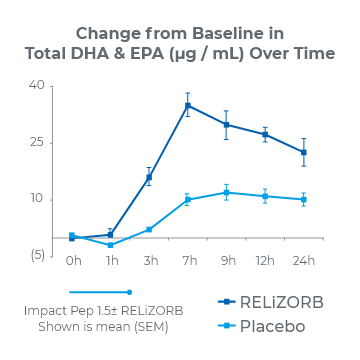
2.8x overall increase in total DHA and EPA plasma concentrations
68% reduction in the incidence of diarrhea
90-Day Study2
(P<0.001)
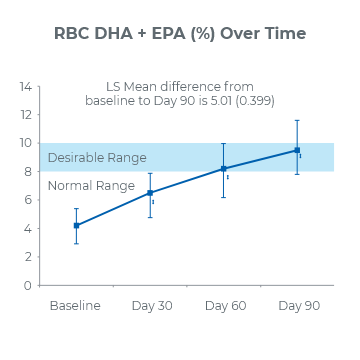
2.1x increase of DHA and EPA in red blood cell membranes
0% reported incidence of diarrhea at Day 90
Therapeutic Targets
Alcresta Therapeutics has developed a novel enzyme-based (iLipase) platform to address the challenges of fat malabsorption faced by people living with serious or rare diseases, such as cystic fibrosis, pancreatitis, short bowel syndrome, and other conditions associated with fat malabsorption. Fat malabsorption has devastating consequences and is caused by the impaired secretion of the pancreatic enzyme lipase, usually associated with exocrine pancreatic insufficiency (EPI), or with changes in gastric, duodenal or liver physiology. Lipase enzymes are essential to the hydrolysis and absorption of dietary fats, especially long chain polyunsaturated fats (LCPUFAs). Conditions commonly associated with fat malabsorption include3,4::
- Cystic fibrosis
- Acute/chronic pancreatitis
- Abdominal surgery
- Short bowel syndrome
- Neonatal intensive care unit (NICU)
- Trauma/critical care
- Pancreatic cancer and other cancers/treatments
Alcresta is uniquely positioned to be the leader in the fat malabsorption market in underserved rare and orphan disease populations. Most of the studies conducted to date have focused on cystic fibrosis and pancreatitis. However, other indications associated with fat malabsorption will continue to be studied.
References:
- Freedman S, Orenstein D, Black P, Brown P, McCoy K, Stevens J, Grujic D, Clayton R. Increased Fat Absorption From Enteral Formula Through an In-line Digestive Cartridge in Patients With Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 2017;65:97-101. https://doi.org/10.1097/mpg.0000000000001617
- Stevens J, Wyatt C, Brown P, Patel D, Grujic D, Freedman SD. Absorption and Safety With Sustained Use of RELiZORB Evaluation (ASSURE) Study in Patients With Cystic Fibrosis Receiving Enteral Feeding. J Pediatr Gastroenterol Nutr. 2018 Oct;67(4):527-532. https://doi.org/10.1097/mpg.0000000000002110
- MedLinePlus Website. https://medlineplus.gov/ency/article/000299.htm
- Haupt ME, Geller DE, Hall JA, Quintana Diez PM. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017;23(39):7059-7076. https://doi.org/10.3748/wjg.v23.i39.7059